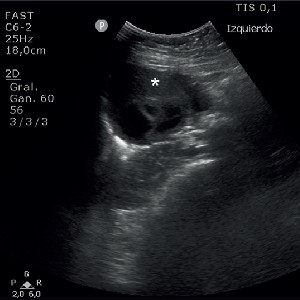
Wearable defibrillator use in patients with high risk of sudden cardiac death: a brief report from a single center

Submitted: 4 April 2023
Accepted: 26 June 2023
Published: 11 July 2023
Accepted: 26 June 2023
Abstract Views: 316
PDF: 130
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- Rodrigo Diaz, Marta Velia Antonini, Distal perfusion cannula on VA-ECMO: do not blindly trust! A case report , Acute Care Medicine Surgery and Anesthesia: Vol. 1 No. 1 (2023)
- Martina Tocco, Alessandra Venditto, Emmanuel Gasperoni, Elisabetta Rinaldi, Emanuele Russo, Vincenzo Tiberio, interspecies competition and infection control strategies , Acute Care Medicine Surgery and Anesthesia: Vol. 1 No. 1 (2023)
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/amsa.2023.14
https://doi.org/10.4081/amsa.2023.14