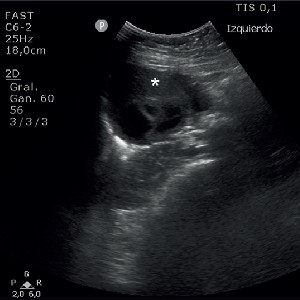
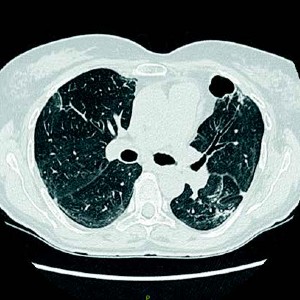
Acinetobacter cavitations in COVID-19 interstitial pneumonia: a case report and review of the literature
Submitted: 3 March 2023
Accepted: 10 March 2023
Published: 6 April 2023
Accepted: 10 March 2023
Abstract Views: 342
PDF: 135
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- Fara Russo, Maria Carolina Russo, Camillo Candurro, Stroke in Mycoplasma pneumoniae pneumonia: a clinical case , Acute Care Medicine Surgery and Anesthesia: Vol. 1 No. 1 (2023)
- Lorenzo Viola, Giuliano Bolondi, Carlo Bergamini, Luca Bissoni, Marco Benni, Domenico Pietro Santonastaso, Sofia Vitali, Mario Piccinno, Luca Mezzatesta, Giovanni Scognamiglio, Alessandro Circelli, Esophageal manometry: a valuable tool for the comprehensive management of COVID-19-related hypoxic respiratory failure , Acute Care Medicine Surgery and Anesthesia: Vol. 1 No. 1 (2023)
- Francesca Schettino, Francesco Coletta, Crescenzo Sala, Antonio Tomasello, Anna De Simone, Elena Santoriello, Marco Mainini, Simona Cotena, Silvia Di Bari, Romolo Villani, Serratus plane block and erector spinae plane block in the management of pain associated with rib fractures in chest trauma: a brief report from a single-center , Acute Care Medicine Surgery and Anesthesia: Vol. 1 No. 1 (2023)
- Gianmarco Russo, Moana R. Nespoli, Dario Maria Mattiacci, Dario Amore, Maurizio Ferrara, Domenico P. Santonastaso, Francesco Imperatore, Marco Rispoli, Thoracic paravertebral block vs mid-point transverse process to pleura block in thoracic surgery. Preliminary evaluation of effectiveness and safety , Acute Care Medicine Surgery and Anesthesia: Vol. 1 No. 1 (2023)
- Vincenzo Pota, Francesco Coletta, Angela Visconti, Giulia Landi, Maria Caterina Pace, Clinical data transmission in intensive care unit: what have we learned from COVID-19? , Acute Care Medicine Surgery and Anesthesia: Vol. 1 No. 1 (2023)
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/amsa.2023.10
https://doi.org/10.4081/amsa.2023.10